Fecal microbiota transplantation (FMT) is the process of administering a preparation of healthy donor stool to a patient with a certain disease, usually Clostridium difficile colitis, in an attempt to treat the disease. I covered some of the basics about the microbiome and FMT in a previous article, so this will just be a cookbook-style post on how we do FMT with colonoscopy.
First, a healthy donor must be identified. The donor should be in good general health, since theoretically some problems such as obesity, diabetes, autoimmune disease, etc., may be transmitted by fecal transplant. The donor cannot have taken antibiotics for at least three months prior to the fecal transplant. The donor must also have appropriate blood and stool tests done (which will be covered in a future article.) It can take several days for all the testing to be done and the results received.
Once the donor is fully screened and ready, the date is set for the transplant. If the patient is still taking antibiotics for C. difficile, he or she is instructed to stop these 48-72 hours prior to the transplant, if possible. The patient is then given a standard colonoscopy bowel prep the night before the procedure. This serves two important purposes. First, the colon must be prepped to allow for a safe colonoscopy and allow the doctor to see where he or she is going. Second, in theory, the bowel prep will purge the colon of stool and bacteria, and allow the new donor stool to colonize the patient without much competition from the patient’s diseased former microbiome. After all, the purpose of the FMT is to replace the dysfunctional microbiome with a new healthy one, so it’s best to start with a blank canvas.
On the day of the transplant, the patient arrives to the endoscopy suite fasting with a prepped colon. On the way to the endoscopy suite, the patient is instructed to take 4 mg of loperamide (Imodium) to slow down the colonic transit and hopefully allow the donor stool to dwell in the colon for a longer period of time. The donor must also produce a stool on the day of transplant, and this specimen will be used to perform the actual transplant. If the donor is not “regular” and cannot be certain that they will be able to move their bowels on the day of the procedure, then a gentle laxative such as milk of magnesia is recommended the evening prior to the transplant, just before going to bed. The donor stool must be provided as fresh as possible, and should be used within 8 hours (maximum) of being passed. Therefore, the donor is instructed to have the bowel movement at home, and to provide the stool in a clean “tupperware” container. Kudos to the few donors who can arrive to the endoscopy suite and have a bowel movement on command just before the procedure, but this is not necessary (nor is it recommended) since sometimes performance anxiety sets in, and then we have a patient and doctor waiting to do the transplant, but no donor stool to do it with!
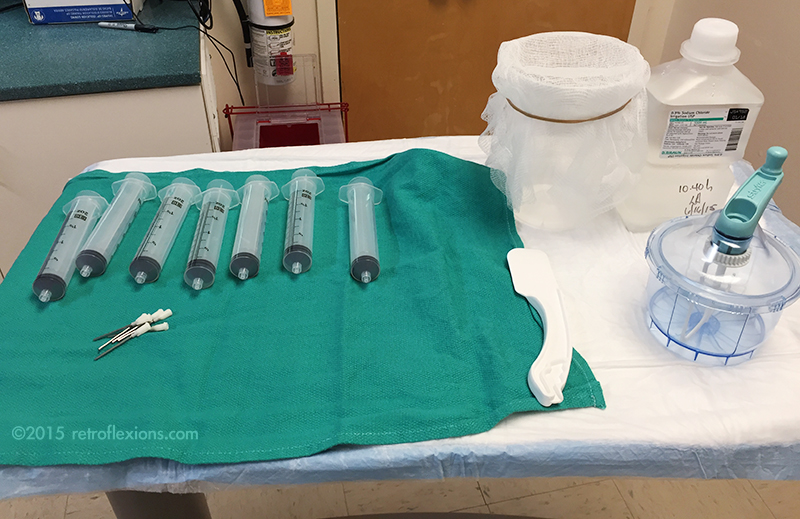
It should go without saying, but obviously full barrier precautions are needed when dealing with stool. The person doing the stool preparation should be wearing a gown, gloves, a mask, and a face shield. If available, this should ideally be done under a laboratory hood with ventilation. The donor stool is prepared as follows:
A mixer is needed; I use a small disposable hand mixer with a tightly-locking lid (made by Stryker to mix bone cement for orthopedic cases…as it turns out, this is a perfect device for mixing stool for FMT.) The entire stool is put into the mixer along with normal saline. I usually start with about 200-300 cc of room temperature non-bacteriostatic saline for the first extraction. The stool and saline is mixed for a minute or two (this is why it is important to have a mixer with a tightly sealing lid) and the result is a brown thin liquid. The mixer is then opened and the liquid is poured slowly into the container with the gauze on top. A rubber band is useful to hold the gauze in place. This process strains out any solid material in the stool, and the result is a brown liquid in the container below.
The solid material captured on the gauze is then put back into the container using the spatula, and another ~200 cc of saline is added. The mixing and straining process is repeated until about 400-500 cc of stool extract is obtained in the container. The extract should be opaque and the color should be a medium-to-dark brown, like very muddy water. If it is transparent, then too much saline or not enough stool was used.
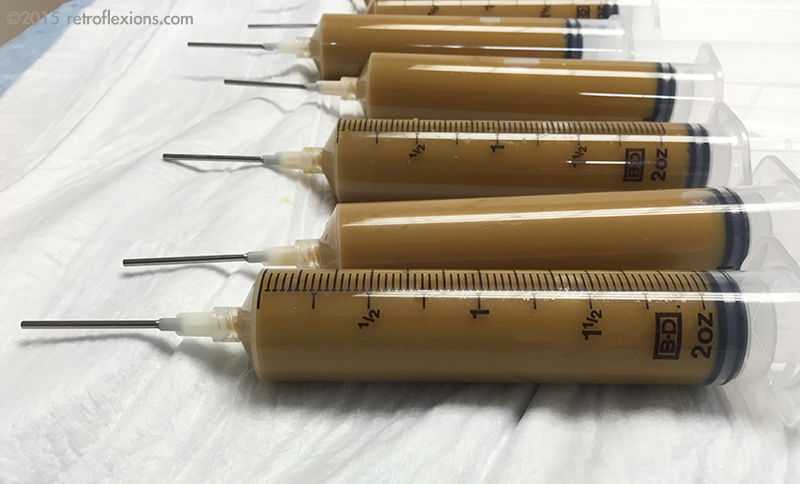
The mixture is then drawn up into large syringes and fitted with blunt-tipped needles made to fit into the colonoscope biopsy port. Each syringe holds 60 cc of material.
The patient is then brought into the procedure room and given the standard sedation used for a colonoscopy. I use carbon dioxide insufflation instead of air for fecal transplant cases since this is easier to absorb and may cause less of the need to pass air (and as a side effect, stool) at the end of the procedure. The colonoscopy is performed and I try to suction out as much as the remaining liquid inside the colon as possible on the way in. Once the end of the colon is reached it is time for the transplant to happen. The entire payload of stool extract (usually 400-500 cc) is delivered into the cecum by injecting the contents of the syringes through the biopsy channel of the colonoscope.
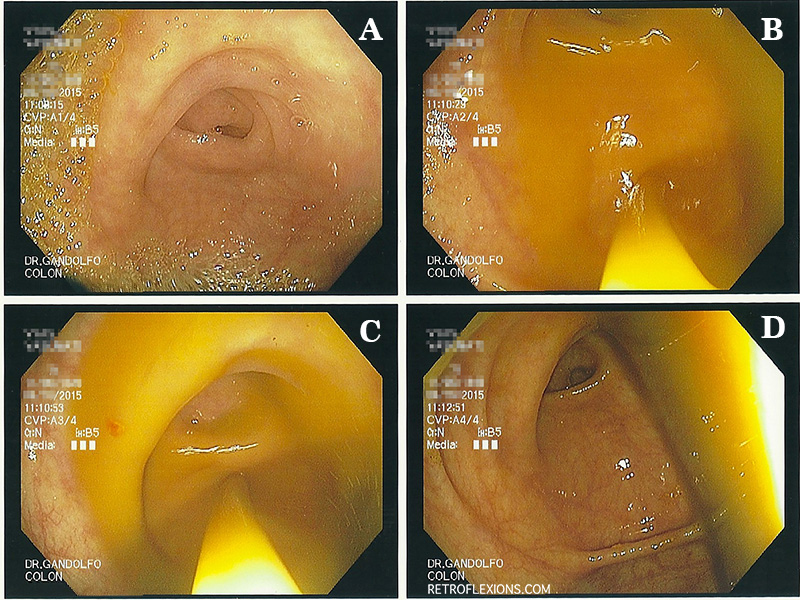
A: appendiceal orifice. B and C: spraying the fecal transplant extract into the cecum through the scope. D: transplanted stool in cecum.
The fecal extract exits the tip of the scope and fills the cecum, and soon will naturally move distally to coat the entire colon. At this point, my goal is to get the scope out as quickly as possible, and not to have any of the transplanted stool follow me out. Gastroenterologists are trained to inspect the colon on the withdrawal phase of colonoscopy, however this must be avoided, and the scope should simply be removed from the patient rapidly without giving any additional insufflation gas in the process. The exception is that if there is a large amount of retained gas in the patient from the procedure, this should be suctioned out quickly. But don’t stop to biopsy a polyp! This being said, it is considered safe to biopsy things on the way into the colon, before the transplant is given.
Remember the Imodium we gave the patient before the procedure? Hopefully this has taken effect by now and slowed down the gut motility giving the transplanted microbiome more time to colonize the colon. The more contact time between the transplanted stool and the patient’s colon the better. The body’s natural tendency is to expel the donor stool, so often despite all of the above efforts, the patient will have watery diarrhea soon after the transplant procedure. The goal is to minimize this as much as possible.
After the transplant, the patient is educated about what to expect over the following days. The occurrence of a low-grade temperature the evening of transplant is not totally uncommon after FMT, and should NOT be treated with antibiotics since this will wipe out the transplant! Antibiotics of any kind should be avoided for as long as possible (unless needed for severe infections.) Constipation, gas, bloating, and excessive flatulence are also sometimes reported in the few days following FMT, but usually these side-effects are mild and self-limited. Probiotics are often given for a month or so after FMT, although this is optional.
Also, it is very important to have the patient’s family prepare the house for their arrival. It needs to be cleaned to prevent re-infection from C. difficile spores, as outlined in a previous article.
If you enjoyed this article, sign up for our free newsletter and never miss a post!
References:
Brandt LJ. American Journal of Gastroenterology lecture: Intestinal microbiota and the role of fecal microbiota transplant (FMT) in the treatment of C. difficile infection. Am J Gastroenterol 2013;108:177-85.
Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: Indications, methodologies, mechanisms, and outlook. Gastroenterology 2015;149:223-237.